
Home Remedies For Hiccups (That Actually Work!)
The next time you get a case of the hiccups, find relief—fast—with these proven home remedies.

The Best Home Remedies For Hemorrhoids
No prescription? No problem. Find relief from the pain and itching of hemorrhoids with these home remedies.

What You Need to Know About Canada’s Three-Layer Face Mask Guideline
Canadian health officials say masks meeting these criteria provide the best protection against COVID-19.

Highly Effective Home Remedies for Constipation
Constipation relief may be as close as your kitchen! These natural home remedies will help get your bowels back on...

How to Do CPR: 7 Essential Steps of CPR Everyone Should Know
Performing CPR right away can double or even triple a person’s chance of surviving cardiac arrest. Take a refresher course...

The Best Home Remedies for Toothache Relief
Whether you're suffering from a cracked tooth or a painful cavity, these home remedies can provide relief while you wait...

“Dark Room” Concussion Treatment Was Wrong—Here’s a Better Way to Recovery
Doctors once prescribed resting in the dark as a treatment for concussions. But recent research shows there is a better...

Yes, Your Seasonal Allergies Are Getting Worse
Climate change is exacerbating seasonal allergies for one in four Canadians who dread the first sniff of spring. Here's how...

A Lyme Disease Vaccine Is Coming—Here’s What You Need to Know
In the meantime, here's how to protect yourself from ticks.

Is That a Doctor in Your Pocket?
This 18-year-old developed an AI-powered diagnostic app that is like a Shazam for symptoms of a rare heart condition.

Oh, No: Vertigo! Here’s How to Handle It.
If your world is spinning, you're not alone: vertigo is more common than you think. Here's how to manage your symptoms.
8 Ways Stress Wrecks Your Body
Stress doesn't just affect your mental health. Your entire body feels it.

13 Silent Signs of Dementia (and How to Spot Them Early)
From sudden personality changes to poor financial decision-making, here are the 13 early signs of dementia you should never ignore.

The Truth About Fatty Liver Disease
Thought fatty liver disease was only a concern for heavy drinkers? Think again. Here's what you need to know about...

What It Could Mean if Your Hands are Always Cold
The underyling cause could be benign—or a symptom of a more serious condition.

Name That Rash: 6 Common Skin Conditions (and How to Treat Them)
You know it's a rash, but what's causing it? Here’s how to tell a patch of psoriasis from a bout...

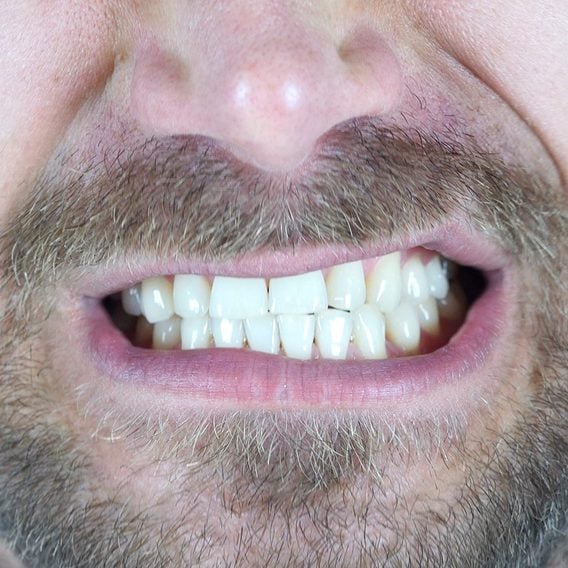
7 Signs of Disease Your Teeth Can Reveal
Dentists are trained to spot more than just cavities: these red flags may signal a health issue happening elsewhere in...

The Best Home Remedies for Nausea
Feeling queasy? Whether it's motion sickness, the side effect of a medication or the dreaded flu, these kitchen cabinet cures...

For Years, Her Excruciating Stomach Pain Would Strike at Random—And Doctors Were Stumped
Every time Genevieve made it to the ER, her debilitating symptoms would vanish as quickly as they'd appeared...

These Are the Heart Attack Symptoms That Are Most Frequently Misdiagnosed
Each year, 63,000 Canadians have a heart attack, and many of them are first told by doctors that the problem...

This Teen’s Vision Loss Was a Mystery—Until a Doctor Asked Him What He Ate
For years, Jerome was losing his ability to see and hear, and became easily exhausted. Could his bad eating habits...

What It Could Mean If You’re Always Waking Up at Night to Pee
Waking up to pee once every night is normal. Anything more than that could be a red flag for these...
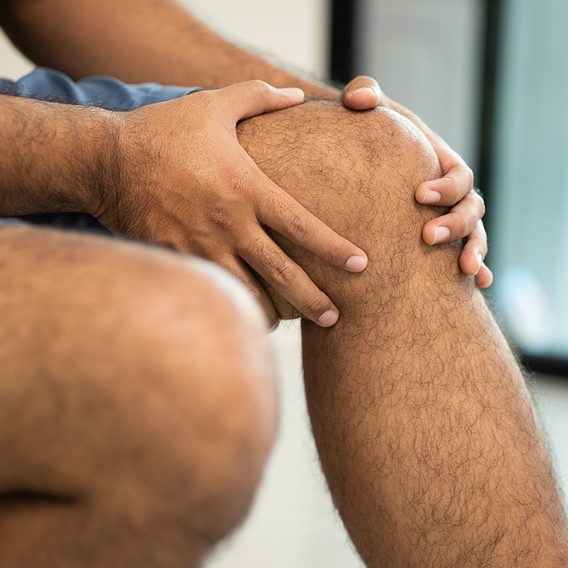
Arthritis Myths You Need to Stop Believing
These common misconceptions about arthritis could be keeping you on the sidelines of a vibrant, active life.

She Was Sure Her Symptoms Were Lyme Disease—But the Bull’s Eye Rash Never Appeared
Vera thought she felt a tick bite her shoulder, but never saw the actual tick. Would the doctors take her...

Stress Poop is Real—Here’s Why it Happens
Anxiety got you headed to the bathroom in a hurry? Find out if it’s just frazzled nerves—or something else.

This Young Woman Figured She Only Had a UTI—Until Her Kidneys Started Failing
Read the incredible true story of how doctors stopped at nothing to solve a medical mystery.

Have Chronic Back Pain? You Could Be Dealing With Axial Spondyloarthritis
If you’re stiff, sore or swollen, it’s time to find the cause.

Silent Signs of Hearing Loss You Might Be Ignoring
If these symptoms sound familiar, it might be time to speak with your doctor.

Subtle Signs of Disease Your Feet Can Reveal
Are your tootsies trying to tell you something? It turns out the condition of your feet can alert you to...
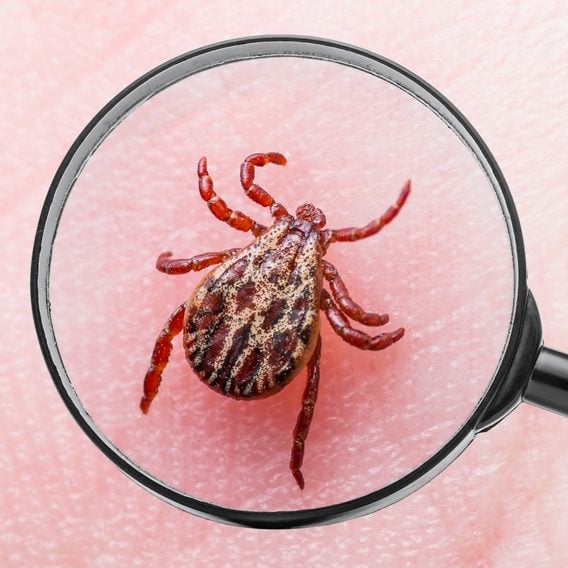
What Everyone Gets Wrong About Ticks
These common misconceptions could put your health at risk.
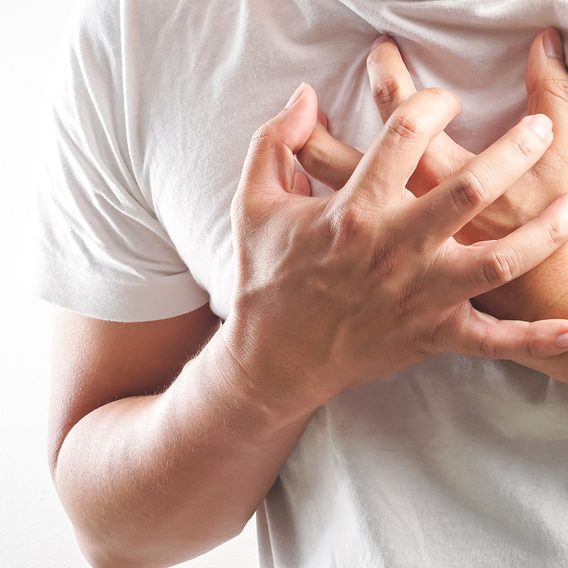
Most Heart Attacks Happen on This Day of the Week
New research might suggest your choices over the weekend could impact your cardiovascular health.